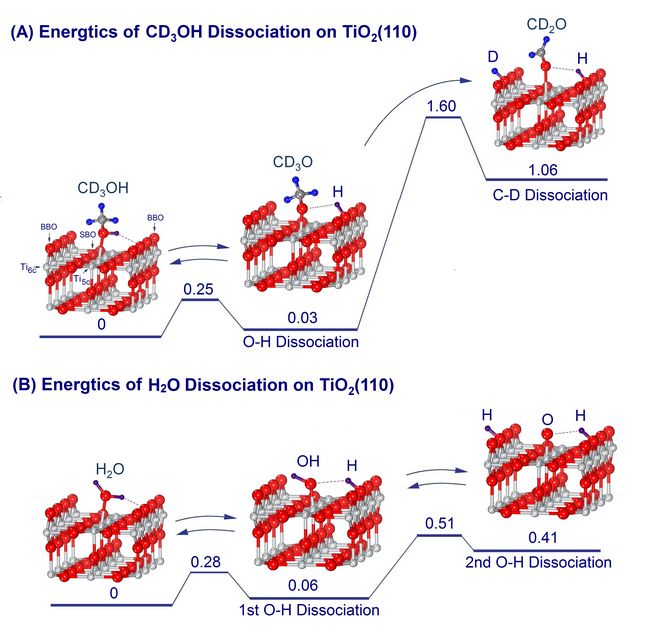
The DICP research group of “surface photochemical reaction kinetics” led by Prof. Xueming Yang has studied the variation in TPD profiles of reactants and products from a TiO2(110) plane covered with methanol and radiated by ultraviolet rays. The surface photochemical reaction kinetic device employed in this study, basing on a high-sensitivity mass spectroscope, was developed by this research team. Their findings showed that in the surface process, methanol was dissociated into formaldehyde, and the dissociation process went through the steps of breaking of the O-H and C-D bonds, and migrating of the H/D atoms to the neighboring bridging O atoms. Moreover, their experimental results were consistent with those calculated via the DFT theoretical method. Their results were published in a paper titling “Stepwise Photocatalytic Dissociation of Methanol and Water on TiO2(110)” on the recently appearing Journal of American Chemical Society (J. Am. Chem. Soc., 2012, 134 (32), pp 13366–13373).
These research accomplishments have important significance in photo-catalysis research on TiO2. It is known that in industrial catalytic decomposition of methanol, the first step is the formation of formaldehyde and hydrogen. However, the results of the DICP team showed that H atoms alone were remaining on the TiO2(110) plane, and this could provide explanation for the fact that low hydrogen productivity was observed when water was photo-dissociated on TiO2. The DICP scientists also found that the 5-coordinated Ti atom possessed photo-catalytic activity, and this important role played by the Ti atom has not been recognized so far.