The DICP Research Group No. 1102 led by Prof. XuemingYang has carried out a systematic study on strong photon energy dependence of the photocatalytic dissociation rate of methanol on TiO2(110). Their results were published in the recent issue of the Journal of American Chemical Society. (J. Am. Chem. Soc., 2013, 135 (50), pp 19039–19045)
In an ideal photocatalyst, all photon energy invested in the generation of charge carriers would be available for redox chemistry. The higher the potential energy of the electron (or hole) the more reductive (or oxidative) capacity there is. But charge carrier thermalization is rapid. As a result, excess potential energy is lost to the lattice via strong coupling with phonon modes, thus reducing the potential advantage gained by the specificity in the absorption event, and the reaction of the adsorbate is thus only dependent on the number of electron-hole pairs created by photo-excitation.
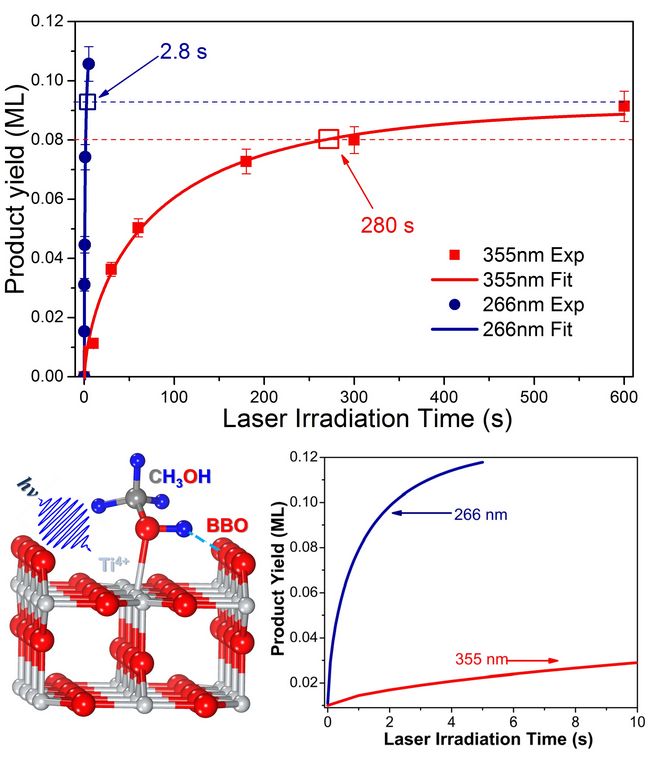
In a recent study, we have shown that photocatalytic dissociation of CH3OH on TiO2(110) occurs in a stepwise manner in which the O-H dissociation proceeds first and is then followed by C-H dissociation to form CH2O with H atoms transferring to bridge bonded oxygen nearby.( J. Am. Chem. Soc., 2012, 134 (32), 13366–13373) As surface temperature increasing, D-atoms on BBO sites (via D2O TPD product) have been detected. In addition to D2O formation by heating the photocatalyzed methanol/TiO2(110) surface, we have also observed D2 product formation. D2 is clearly formed via thermal recombination of the D-atoms on the BBO sites from photocatalysis of methanol. Experimental results indicate that D2O formation is more important than D2 formation and that D2 formation is clearly affected by the D2O formation process.(J. Am. Chem. Soc., 2013, 135 (28), 10206–10209). In this work, photocatalytic dissociation of methanol (CH3OH) on a TiO2(110) surface has been studied by temperature programmed desorption (TPD) at 355 nm and 266 nm. The dependence of the reactant and product TPD signals on irradiation time has been measured, allowing the photocatalytic reaction rate of CH3OH at both wavelengths to be determined. The initial dissociation rate of CH3OH at 266 nm is nearly two orders of magnitude faster than that at 355 nm, suggesting that CH3OH photocatalysis is strongly dependent on photon energy. This experimental result raises doubt about the widely accepted photocatalysis model on TiO2, which assumes that the excess potential energy of charge carriers is lost to the lattice via strong coupling with phonon modes by very fast thermalization, and the reaction of the adsorbate is thus only dependent on the number of electron-hole pairs created by photo-excitation. (By Chenbiao Xu and Qing Guo)