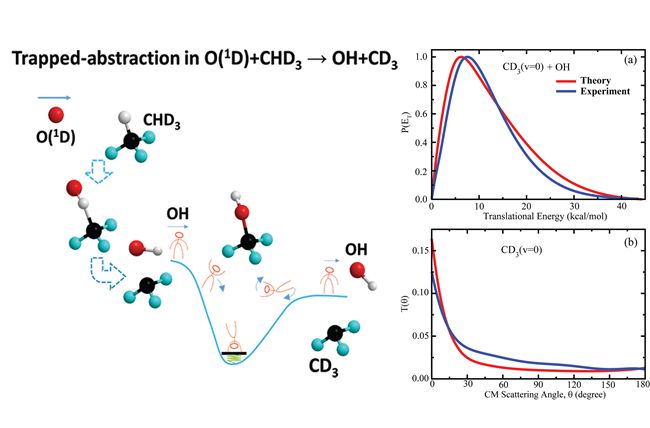
Despite significant progress made in past decades, it is still challenging to elucidate dynamics mechanisms for polyatomic reactions, in particular involving complex formation. The reaction of O(1D) with methane has long been regarded as a prototypical polyatomic system of direct insertion reaction in which the O(1D) atom can insert into the C-H bond of methane to form a “hot”methanol intermediate before decomposition.
Recently, a combined theoretical and experimental study on the O(1D)+CHD3→OH+CD3reaction was carried out by a DICP team led by Prof. Xueming Yang and Prof. Dong H. Zhang, on which good agreement between theory and experiment is achieved. It wasfound that this complex-forming reaction actually proceeds via a new mechanism(they termed it as the trapped abstraction mechanism), rather than insertion mechanism as has long been thought. Detailed analysis revealed that the reaction initially takes place through the O(1D) abstraction of H atom out of CHD3, instead of inserting into the C-H bond. The consequently formed CD3OH complex is easy to dissociate because the energy released from complex formation is highly localized in relative motion of OH and CD3. It is anticipated that this new reaction mechanism should also be responsible for the reaction of O(1D) with ethane and propane, as well as many other chemical reactions with deep wells in the interaction region.
The paper named“Trapped Abstraction in the O(1D)+CHD3→OH+CD3 Reaction”was recently published in The Journal of Physical Chemistry Letters(J. Phys. Chem. Lett.2014, 5, 3106-3111), written by Bina Fu, Dong. H Zhang and Xueming Yang etc. (By Bina Fu & Jiayue Yang)