A paper with the title of "CH Stretching Excitation in the Early Barrier F + CHD3 Reaction Inhibits CH Bond Cleavage" was published in the Science journal not long ago (Science 325, 303-306, 17 July, 2009). The first author of the paper was ZHANG Weiqing, a Ph.D. student of DICP working at present at the Institute of Atomic and Molecular Sciences(IAMS), Taiwan, as a co-supervised graduate student. In IAMS, this work of Zhang was being carried out under the supervision of Prof. Kuoping Liu, which has revealed very unique and interesting findings concerning the reaction of F+CHD3, and has posed a challenge on the understanding of reaction mechanisms.
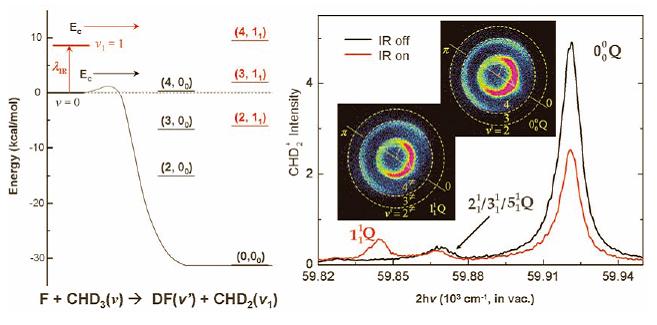
According to the Polanyi Rule summarized by scientists from theoretical and experimental researches of many decades, in a tri-atomic reaction system, the vibration and translation energies of the reactants will exert certain extent of influence to the proceeding of the reaction, and the influence mainly depends on the location of the potential barrier in the reaction path, namely, whether it is a late barrier or an early barrier. Previously, in studies involving reactions of vibration excitation states, researchers have preferentially chosen reactions with late barriers, since it was predicted from the Polanyi Rule that the vibration excitation energy will enhance the occurring of this kind of reactions. As for reactions with early barriers, they have been scarcely studied, because researchers considered that vibration excitation has little effect on these reactions. Is this really the case? The paper of Zhang and co-workers has given a unique and interesting answer.
The research team headed by Prof. Kuoping Liu in IAMS has conducted detailed studies on the F+CHD3 reaction by means of a home-made installation based on crossed molecular beam and time-sliced ion velocity imaging methods. In their experiments, they have found for the first time in history that an increase in the vibration energy has retarded the occurring of the reaction. This implied that the stretching vibration of the C-H bond has controlled strongly the kinetic course of the reaction, which not only inhibited effectively the ravaging of the H atom by the F atom, but also influenced the cleavage of the C-D bond, thus resulting in the change of the branching ratio of the various vibration states of DF.