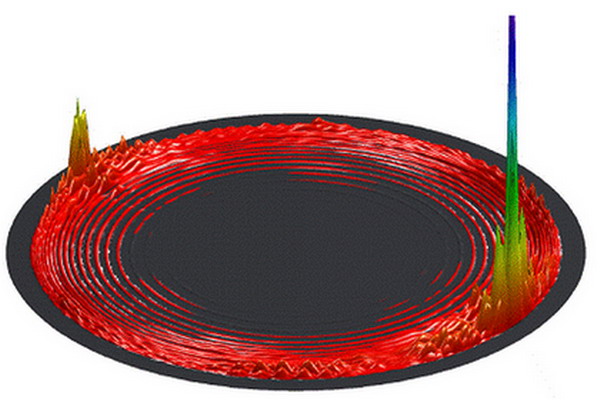
Differential cross sections (DCS) for the O + O2 reaction at collision energy of 0.05eV
(Picture/the State Key Laboratory of Molecular Dynamics)
Important progress in reaction kinetic studies has been made by researchers of the State Key Laboratory of Molecular Dynamics and the Theoretical Center of DICP. A paper with the title of "State-to-state quantum dynamics of O+O2 isotope exchange reactions reveals nonstatistical behavior at atmospheric conditions", written by SUN Zhigang, LIU Lan, ZHANG Donghui and co-workers, was published in the latest issue of the PNAS(Proceedings of National Academy of Sciences, USA).
It is well known that the photo-decomposition of ozone in the atmosphere will absorb most of the ultra-violet(UV) light from the sunshine, thus protecting the creatures of the earth from injuring by the UV light. Meanwhile, new ozone molecules will be regenerated via the following reaction, thus maintaining an equilibrium concentration of ozone in the atmosphere:
O+O2 → O3*→ O+O2
O3*+M →O3+M
It can be seen that in this reaction, the collision of O and O2 can produce highly excited O3* intermediates, which in turn can either re-decompose into O+O2, or collide with another molecule M to form a stable O3. Hence, this exchange reaction of O+O2 is a very important reaction in atmospheric chemistry. However, due to the existence of a very deep potential well of O3*, state-state quantum kinetic studies on this reaction system is unrealized so far.
In 2008, DICP researchers SUN Zhigang, ZHANG Donghui and co-workers developed an effective tri-atomic state-state kinetic method, which was successfully applied to the important reaction of H+O2 in combustion chemistry, and obtained for the first time the state-state differential cross-section of the reaction(Journal of the American Chemical Society 130,14962 (2008)). In 2009, they optimized further this approach, and were able to obtain for the first time a state-state differential cross-section with collision energy less than 0.1eV on the O3 potential surface constructed by Schenke and co-workers. They have also discovered that this oxygen exchange reaction, which involves three heavy atoms, showed a strong nonstatistical effect, and was quite different from the results obtained by Hua Kuo and co-workers of the New Mexico University of USA. It is envisaged that this nonstatistical effect has important significance for the studies of ozone.