Prof. ZHOU Yonggui of DICP, by collaborating with Prof. FAN Hongjun, has realized recently the asymmetric hydrogenation of simple pyrroles for the first time, and their results were published in the newly appeared Journal of American Chemical Society(Journal of the American Chemical Society, 2011, 133, 8866-8869).
In recent years, Prof. Zhou s research team has been putting great efforts in developing new activation tactics for the asymmetric hydrogenation of aromatic compounds. By means of substrate/catalyst activation, they have succeeded in asymmetric hydrogenation of aromatic heterocyclic compounds such as quinolines, iso-quinolines, functionalized pyridines, quinoxalines, and simple indoles.
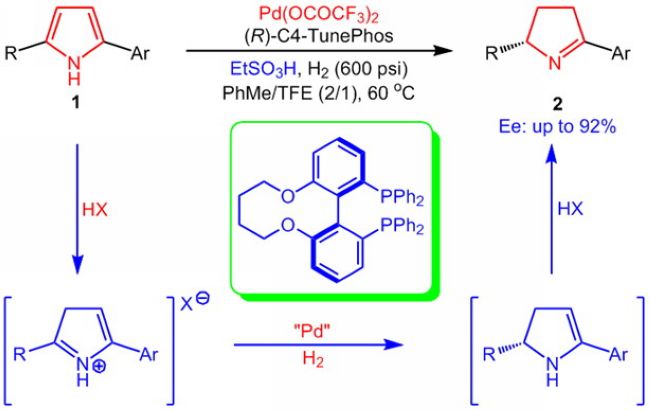
In recent studies, they have activated pyrroles via protonation of the double bond by Bronsted acids, then followed by asymmetric hydrogenation over a palladium catalyst. The products were partly hydrogenated chiral 1-pyrroline derivatives, and the maximum enantiotropic selectivity reached 92%. Moreover, the products contained an imine group which favored subsequent conversions, and provided a convenient and simple new route for these kind of reactions. The researchers also gave rational explanations for the research results on the basis of theoretical computation and isotope labeling experiments.