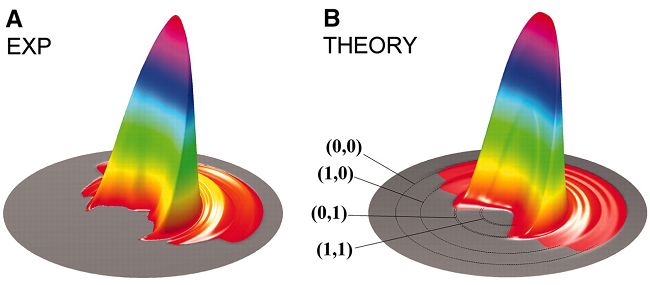
The reaction system H2 + OH → H2O + H is a basic model system in four-atom reactions. It is also an important reaction in combustion chemistry and interstellar chemistry. Moreover, its reverse reaction is a swatch reaction in the study of mode-selective chemistry.
In the past few years, Prof. YANG Xueming and Prof. ZHANG Donghui of DICP and their co-workers have conducted kinetic studies on the isotope-substituted reaction of HD + OH → H2O + D. Theoretically, they have developed a set of highly efficient time-dependent wave-packet method, which could be employed for delicate computations of four-atom reactions with six degrees of freedom. Moreover, they were able to construct the potential energy surface of this reaction with a more précised method, thus succeeded for the first time in the accomplishing of a full-dimension quantum-state resolved kinetic computation. Experimentally, they have determined the differential cross-sections at different reaction energies as well as their variation with the colliding energies by means of a high resolution cross molecular beam-Rydberg deuterium TOF spectrometry technique. It was shown that the experimental results agreed excellently with the theoretical computations.
This work was the first one in a four-atom reaction system that has attained high coincidence in theoretical and experimental results of state-state differential cross-sections, and this signified a vital breakthrough in the study of molecular dynamics. This also implied that DICP has been keeping a leading position internationally in the area of molecular dynamic research.
This work was financially supported by the State Ministry of Science & Technology as well as the National Natural Science Foundation of China. The results of this work was published in Science appearing on 22nd July, 2011(Science 333,440(2011)).